Industrielle Mischtechnik Made in Germany
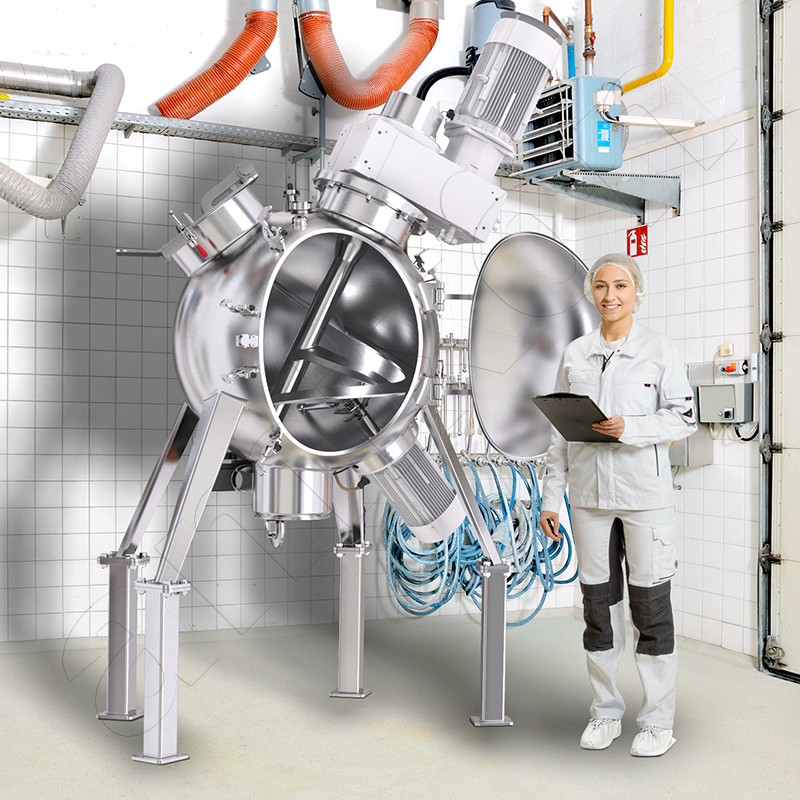
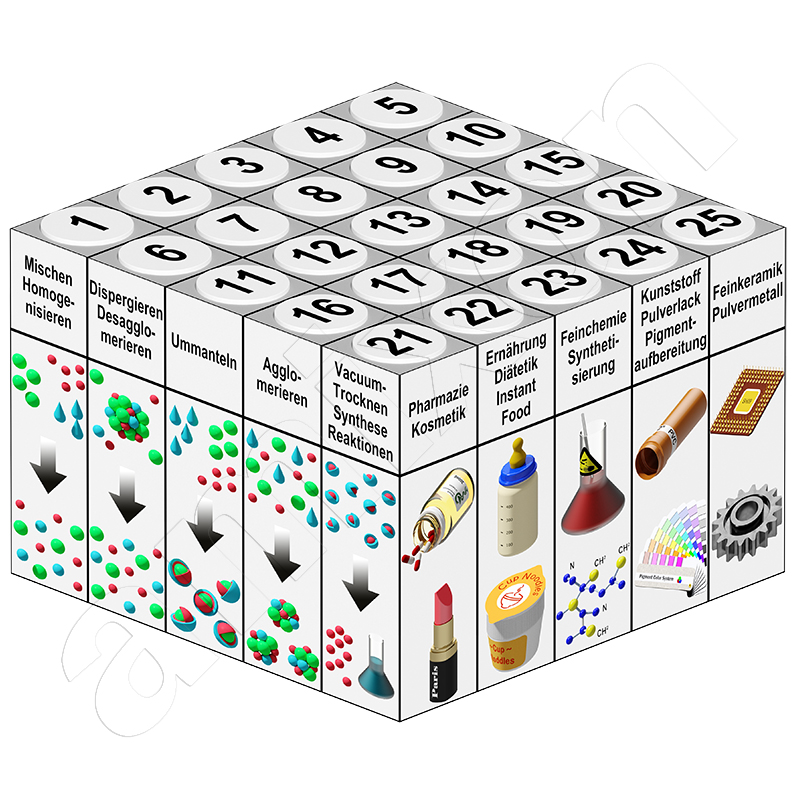
amixon® entwickelt und fertigt seit fast vierzig Jahren hochpräzise Mischanlagen für industrielle Anforderungen. Pulvrige Güter (trocken, feucht oder suspendiert) werden gemischt, veredelt, agglomeriert, getrocknet oder chemisch verändert.
Unser Sortiment umfasst verschiedene Mischanlagen zur Anwendung in der aufbereitenden Industrie: darunter die Lebensmittelindustrie, Pharmazie, keramische Industrie, Pulvermetallurgie und Synthesechemie.
Profitieren Sie von innovativen, maßgeschneiderten Lösungen für Ihr Produkt und Ihre Spezifikationen dank:
- einem möglichen Mischkesselvolumen von 10l bis 50.000l
- modifizierbaren Einstellungen und Verarbeitungsfunktionen
- einer nahtlosen Integration in Ihre Produktionslinie
- Unterstützung bei der Pilotierung, der Validierung und der Zertifizierung.
Erstklassige Leistung und beispiellose Langlebigkeit: amixon® ist Trendsetter für vertikale Mischer und fertigt Mischanlagen für Branchenführer.
Innovative Mischtechnik für internationale Märkte
amixon® Technikum
Besuchen Sie unsere ausgezeichnet ausgestatteten Technika in Deutschland, den Vereinigten Staaten, China, Japan, Indien, Thailand sowie Südkorea und profitieren Sie von unserem Know-How aus Leidenschaft, und das seit 40 Jahren.